Using MyPath in routine cancer care
With the creation of any innovative tool or approach, it is essential to understand how the innovation and accompanying processes are integrated with existing practices. In order for MyPath, our digital solution to patient-centred cancer care, to deliver the desired outcomes, careful planning, iterative system design, and an evidence-informed implementation strategy is key. Therefore, parallel to the development of MyPath, an implementation study will be conducted, in which we investigate how MyPath can best be introduced into current clinical practice and scaled.
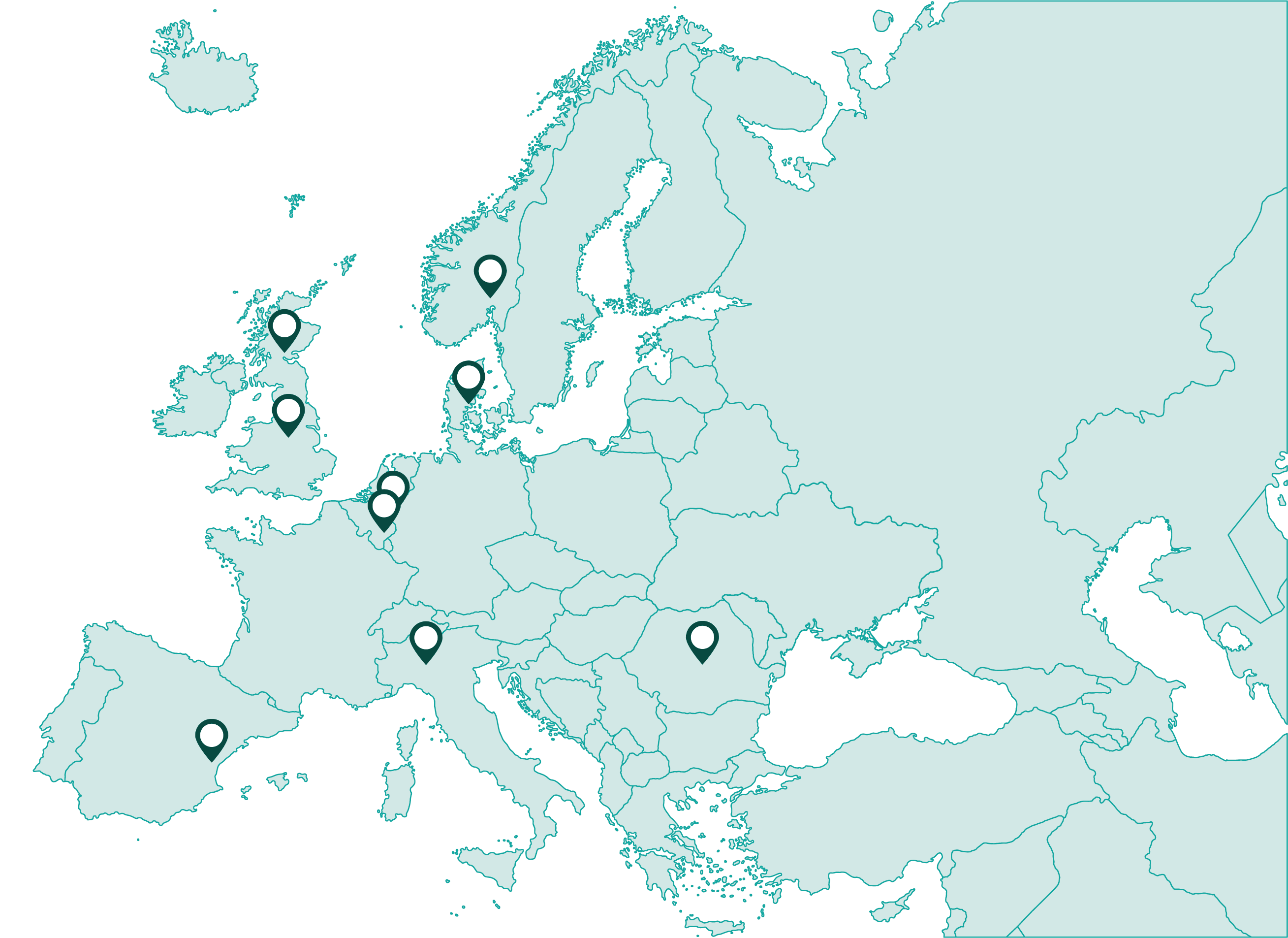
The implementation study involves investigating implementation contexts and the iterative implementation of MyPath at nine clinical centres across Europe. The resulting theory of change will seek to explain what might work, for whom, how, and under what circumstances, informing the appropriate application of MyPath in a wider European context. This will ensure that benefits of the intervention are maximised whilst inadvertent risks are minimised.

It is fundamental that before developing any implementation strategy, we conduct an appropriately designed implementation study. This will inform any strategy and give us the best chance of success.
Prof. Marie Fallon, University of Edinburgh – MyPath project co-lead & implementation and validation lead
In the implementation study, MyPath will be developed with a range of stakeholders and tested and refined in real-world clinical contexts. After pilot trials at selected cancer centres, MyPath will be implemented at all nine cancer centres in our consortium during the main trial. In parallel to these trials, qualitative data collection and analysis will be carried out to gain insights into evolving processes across implementation contexts (including emerging unanticipated consequences). The ultimate goal of the implementation study is to answer the question: How can we best implement a digital patient-centred cancer care pathway as part of standard oncology care?
Development and pilot study
Before conducting the pilot trials in the selected clinical centres, each of the trial contexts needs to be well understood.
In the first phase of the pilot study, we will therefore gather the information needed to develop the intervention. This means that we will explore user (patient and healthcare staff) needs and expectations of the intervention, potential barriers and facilitators to implementation, current work and organisational practices, existing technological/organisational infrastructures, and political and cultural contexts. This information will be collected from the participating cancer centres through qualitative interviews with a wide range of staff and ethnographic observations.
To understand the wider contextual environment surrounding the clinical centres, we will also conduct in-depth interviews with other stakeholders such as system vendors, strategic decision makers, implementation teams, and policymakers.

In the second phase, the pilot trial will be successively conducted in a number of selected cancer centres, starting in Oslo. At the same time, data will continue to be collected from these sites to help us assess how the intervention integrates with existing care processes and cultures and determine contextual differences across settings. This, in turn, will allow us to make final improvements to MyPath and the intervention procedure before the main trial.
Key facts
- 20,000 patients
- Breast, prostate, lung, gastrointestinal, testicular, head and neck cancer
- 9 cancer clinics
- 8 European countries
Main intervention and qualitative process evaluation
The main intervention will be carried out in nine cancer settings across eight countries, representing different healthcare systems, cultures, traditions, and beliefs within Europe. The study population will consist of patients with breast, prostate, lung, gastrointestinal, testicular, or head and neck cancer at all stages, including cured patients. These include the most common types of cancers. We aim to recruit 20,000 patients, who will represent the normal sex and age distribution of cancer diagnoses and outcomes.
We will collect in-depth qualitative data from a subset of three trial sites and test the emerging findings from these in the remaining six settings. This will be done through a combination of multidisciplinary focus groups, qualitative interviews, and non-participant observations. We will collect data longitudinally, to allow us to observe developments over time as the use of MyPath becomes embedded in practice.
The evaluation of the main trial will help us to understand the main trial outcomes and what they mean for the sustainability and the wider adoption of MyPath across Europe.


